Electrochemistry
The Nilar advanced bi-polar Nickel Metal Hydride (NiMH) battery module consists of ten cells in series. Each cell contains two electrodes (a positive and a negative), electrolyte, and a separator in-between the two electrodes. The table below describes the compounds in the cell that are active during charge and discharge of the cell. The electrolyte is involved in the chemical reactions at the electrode surface together with the active material during charge and discharge, but is not consumed by the reactions. The strength of the electrolyte is not changed during any of these modes.
| Charge products | Discharge products | |
| Positive material | Nickel (III) oxyhydroxide (NiOOH) | Nickel (II) hydroxide (Ni(OH)2) |
| Negative material | Metal hydride (MH) | Metal alloy (M) |
| Electrolyte | KOH | KOH |
Charge
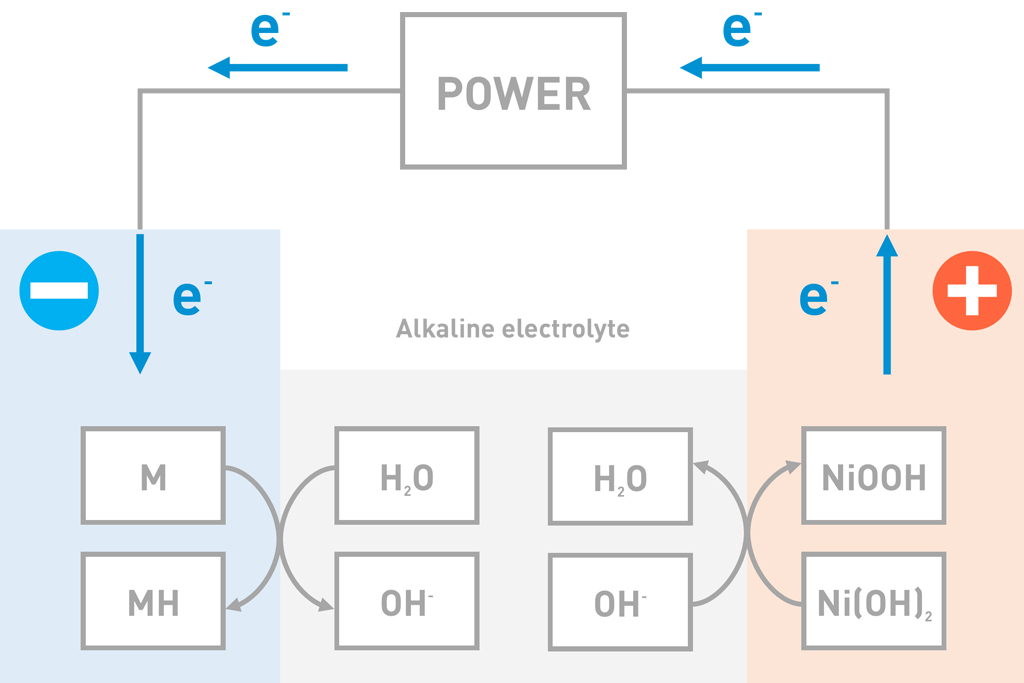
During charge, the hydrogen moves in the opposite direction as compared to the discharge. As the battery is charged, Nickel hydroxide (Ni(OH)2) in the positive electrode lose hydrogen and the metal alloy (M) take up hydrogen to form a metal hydride (MH). When losing hydrogen, the Nickel hydroxide (Ni(OH)2) oxidizes and the positive active material becomes Nickel oxyhydroxide (NiOOH).
| Negative electrode: | M+H2O+e– → MH+OH– |
| Positive electrode: | Ni(OH)2+OH– → NiOOH+H2O+e– |
| Entire battery: | M+Ni(OH)2 → MH+NiOOH |
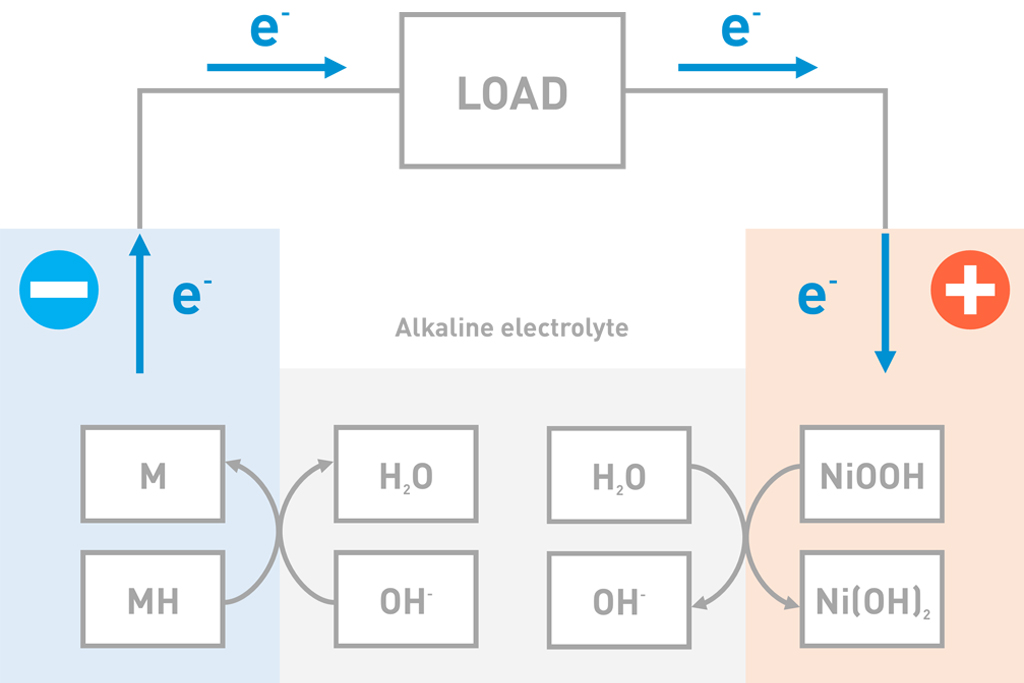
Discharge
When a NiMH battery is discharged, hydrogen moves from the negative active material (MH) to the positive active material (NiOOH). In this process, the metal hydride (MH) is drained of hydrogen and the positive active material is reduced to Nickel hydroxide (Ni(OH)2).
| Negative electrode: | MH+OH– → M+H2O+e– |
| Positive electrode: | NiOOH+H2O+e– → Ni(OH)2+OH– |
| Entire battery: | MH+NiOOH → M+Ni(OH)2 |